Current State Immunization Laws and COVID-19

Introduction
The purpose of this analysis is to summarize the extent to which state laws currently address mandated school vaccinations, exemption policies, and plans for modifications and updates. It also discusses the potential applicability of the existing state laws for COVID-19 vaccination-related requirements for schools and briefly reviews relevant pending legislation in the states.
The state law data were compiled through primary legal research conducted by researchers in the School of Public Health at the University of Illinois Chicago. State laws were compiled using Lexis-Nexis14 and Westlaw15 by trained legal researchers and analyzed by an attorney and trained analysts.
Download the fact sheet
Background
Vaccination mandates are commonplace in United States schools. The individual diseases that require immunization, approved schedule of doses, and in some instances the type of approved vaccines themselves that are necessary for school entry are dictated by each state. At the same time, based on arguments of religious and personal freedoms, many states provide exemptions from school vaccination requirements on an individual basis for closely held religious and/or personal beliefs.
Each year, the federal Advisory Committee on Immunization Practices (ACIP) publishes its recommended childhood immunization schedule.1 ACIP is comprised of both medical and public health experts, and its recommendations are approved by the Director of the Centers for Disease Control and Prevention (CDC) before being published in the CDC’s Morbidity and Mortality Weekly Report.2,3
ACIP meets frequently throughout the year, and closely monitors new vaccines that are in the process of undergoing Food and Drug Administration (FDA) authorization. While FDA approval typically takes years, it can be expedited in the case of Emergency Use Authorizations following the declaration of a public health emergency.4
The introduction and spread of SARS-CoV-2 (COVID-19) across the United States led to the suspension of in-person learning in many school districts across the country beginning in March 2020. The U.S. Department of Health and Human Services (DHHS) first issued a Declaration Under the Public Readiness and Emergency Preparedness Act for Medical Countermeasures Against COVID-19 effective February 4, 2020.5 To date, two COVID-19 vaccines have received emergency FDA authorization for adults (one for ages 16+).6 If and/or when COVID-19 vaccines become available for children, states will be at the forefront of deciding to what extent any new vaccine should be required for school-aged children, whether exemptions will apply, and how to best protect the safety and health of the entire school community.
Results
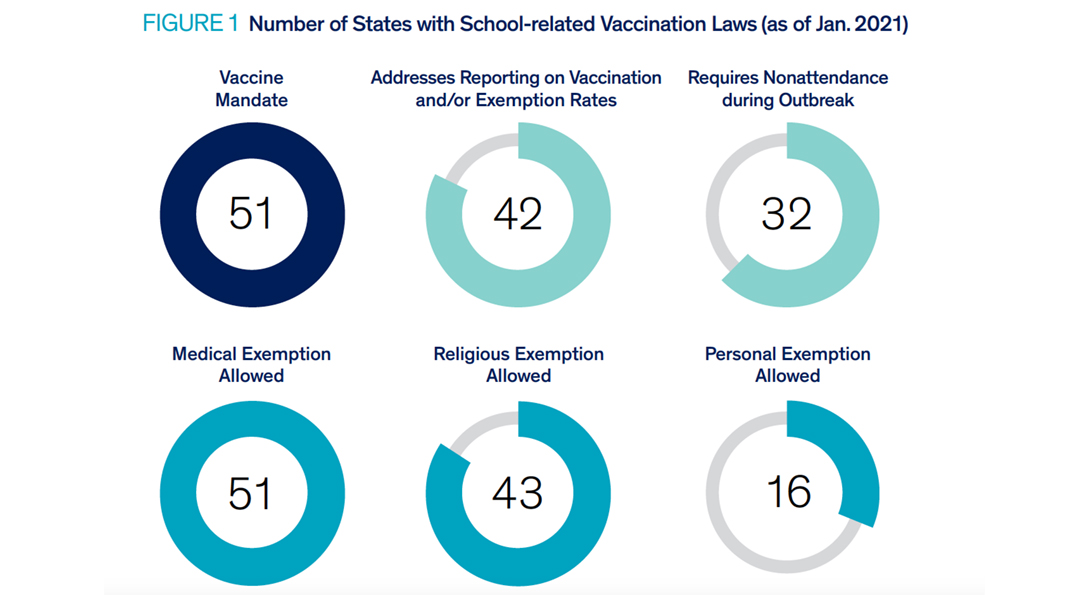
As January 2021, all states and the District of Columbia (D.C.) require certain vaccinations in order to attend school. Some vaccines, such as DTaP (diphtheria, tetanus, pertussis); MMR (measles, mumps, rubella); and polio are required in nearly all states. Fewer states address vaccination for Varicella (Chicken Pox, 40 states); Hepatitis B (39 states); Hepatitis A (15 states); or Human Papillomavirus (6 states). Massachusetts recently mandated the influenza vaccine to begin in 2020, while two other states (Alabama and West Virginia) discuss or otherwise recommend vaccination for influenza.
Laws in 31 states and D.C. require that vaccines follow age-appropriate immunization schedules (as recommended by ACIP) or require boosters in later grades (ex. Tdap). The remaining 19 states’ laws discuss vaccines in terms of entry requirements without specificity. Although 41 states’ and D.C.’s laws address reporting on overall vaccination and/or exemption rates to state health departments for those students who are required to submit paperwork (e.g. students new to the district, 7th graders after receiving boosters, kindergarteners at first entry), only Massachusetts’ influenza policy addresses annual recording and reporting of all students.
Figure 1 illustrates the prevalence of states with laws that provide exemptions from mandatory vaccination requirements. All states and D.C. allow for medical exemptions followed by religious (42 states and D.C.) and personal exemptions (16 states). However, 31 states and D.C. prohibit students from attending school during an outbreak (e.g., measles outbreak in a classroom, school, or community).
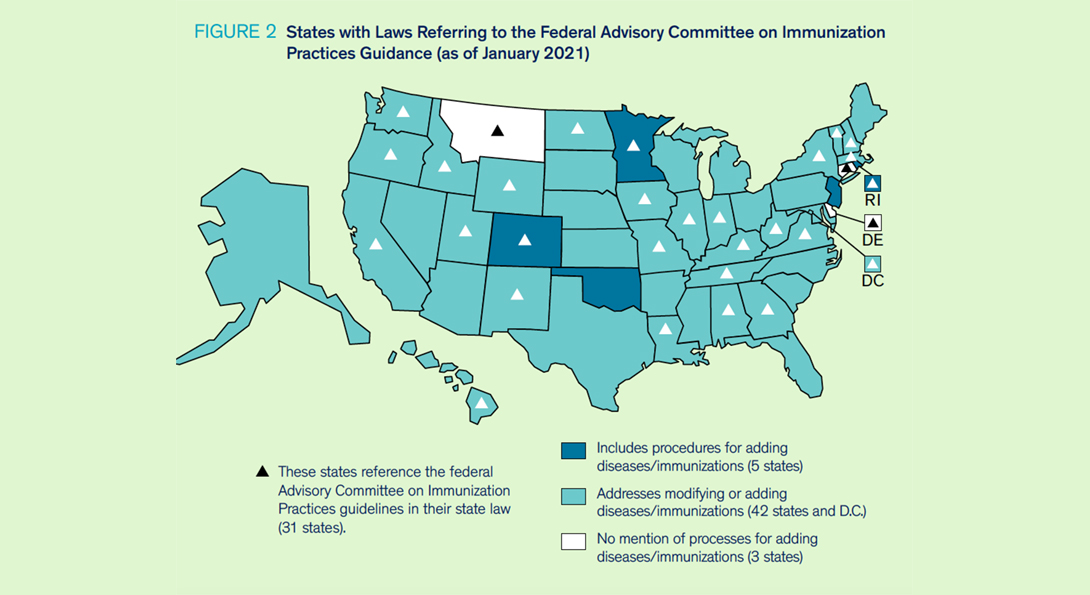
Five states’ laws provide details on the process for adding diseases that require vaccination and/or new immunizations and what it entails (see Figure 2). Forty-two states’ and D.C.’s laws address modifying or adding additional diseases/immunizations but lack specificity on how that process would occur. Thirty states and D.C. reference ACIP in their laws when making decisions related to mandatory vaccinations, while 7 states’ laws reference the American Academy of Pediatrics (AAP) and 10 states’ laws discuss FDA approval.
Legislation specifically addressing COVID-19
Since the emergence of COVID-19, some states have introduced legislation that specifically discusses COVID-19 vaccination for public school students. As of January 23, 2021, 4 states (MN7, NJ8, VA9, and WA10,11) have introduced legislation stating that a COVID-19 vaccine cannot be required for school entry. Washington state also proposed a COVID-19-specific exemption policy.10 A New Jersey bill12 discusses requiring an influenza vaccine while there is no COVID-19 vaccine available for school-aged children in an effort to improve the overall health of the school community. Finally, while not specific to school students, Wisconsin introduced a bill that would limit the ability of its Department of Health to require any person to be vaccinated against COVID-19.13
Conclusion
Many states have not had to revisit their list of mandatory vaccinations for some time, but do rely on the recommendations of the ACIP, AAP, and FDA to make their decisions. Given traditional vaccination schedules and the infrequent use of requiring annual vaccines (such as influenza) in state law, any decision to mandate a COVID-19 vaccine will also require each state to update the time and frequency of collecting annual vaccination information from all students as well as any available exemption policies to date.
About the authors
Elizabeth Piekarz-Porter, JD, is an adjunct instructor of health policy and administration and a research specialist with the Institute on Health Research and Policy, both at the UIC School of Public Health.
Rebecca M. Schermbeck is a research specialist at the Institute on Health Research and Policy at the UIC School of Public Health.
Jamie Chriqui, PhD, is a professor of health policy and administration at the UIC School of Public Health.
Acknowledgement
Support for the state law compilation was provided under a subaward from Child Trends (#4017.UIC.01; prime sponsor: Robert Wood Johnson Foundation). The views and opinions expressed in this fact sheet are solely those of the authors and do not necessarily reflect those of the University of Illinois Chicago, the Policy, Practice, and Prevention Research Center, or the funder or their sponsors.
References
- Centers for Disease Control and Prevention, ACIP Recommendations. 2021. Accessed January 26, 2021.
- Robinson, C.L. Bernstein, H. Poehling, K. Romero, J. Szilagyi, P. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger- United States, 2020. MMWR Morb. Mortal Wkly Rep. 2020;69(5): 130-132. doi: 10.15585/mmwr.mm6905a3.
- Centers for Disease Control and Prevention, Recommended Child and Adolescent Immunization Schedule 2020. 2020. Accessed January 26, 2021.
- Authorization for medical products for use in emergencies. 21 U.S.C. 360bbb-3 (2020).
- Department of Health and Human Services. Declaration Under the Public Readiness and Emergency Preparedness Act for Medical Countermeasures Against COVID-19. Fed. Reg. 2020;85(52): 15198-15203. Accessed January 26, 2021.
- Food and Drug Administration. COVID-19 Vaccines. 2021. Accessed January 26, 2021.
- H.B. 261, 92nd Reg. Sess. (Minn. 2021).
- A.B. 5096, 219th Leg. (N.J. 2020).
- H.B. 2242, 2021 Leg. Sess. (Va. 2021).
- H.B. 1065, 67th Leg., Reg. Sess. (Wash. 2021).
- S.B. 5144, 67th Leg., Reg. Sess. (Wash. 2021).
- A.B. 4576, 219th Leg., (N.J. 2020).
- A.B. 1, 105th Leg., Reg. Sess. (Wis. 2021).
- LexisNexis. Lexis Advance. Accessed January 23, 2021.
- Thomson Reuters. Westlaw Edge. Accessed January 23, 2021.